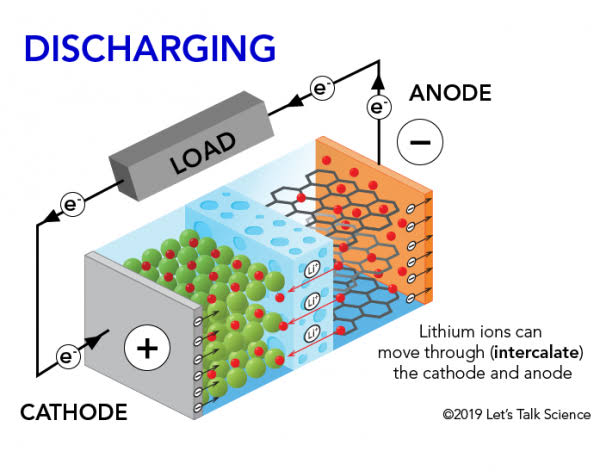
- The discharge of the battery is actually an oxidation-reduction reaction
When a lithium-ion battery is discharged, the lithium ions move from the negative electrode (anode) to the positive electrode (cathode) through an electrolyte solution. This movement of ions creates a flow of electrons that can be harnessed as electrical energy. The discharge process involves an oxidation-reduction reaction in which lithium atoms lose electrons at the anode and become positively charged ions while oxygen atoms gain electrons at the cathode and become negatively charged ions.
During charging, this process is reversed, with the lithium ions moving back to the anode and being incorporated into a graphite matrix while oxygen is released from the cathode. The battery can then be recharged and used again.
The efficiency of this process depends on several factors such as temperature, current density, and cell design. Over time, however, repeated cycles of discharging and recharging cause degradation to occur in both electrodes. As a result, batteries gradually lose capacity until they are no longer able to hold enough charge for practical use.
- Movement of electrons during discharge
During discharge, lithium ions move from the anode to the cathode through the electrolyte while electrons flow through an external circuit. The movement of these electrons generates a current that can be used to power devices. As lithium ions move to the cathode, they react with cathode materials such as cobalt oxide or iron phosphate, releasing energy and forming lithium compounds.
The movement of electrons during discharge is facilitated by the presence of conductive materials in both the anode and cathode. In most lifepo4 battery products , graphite is used as a conductive material in the anode while metal oxides such as cobalt oxide or iron phosphate are used as conductive materials in the cathode. These materials allow for efficient transfer of electrons between electrodes during discharge.
Overall, understanding how electrons move during discharge is crucial for optimizing battery performance and improving battery technology. By studying this process in detail, researchers can develop new materials and designs that enable faster charging times, longer battery life, and higher energy densities.
- Movement of electrons during charging
During charging, lithium ions move from the cathode to the anode while electrons move in the opposite direction. The movement of these electrons is facilitated by a circuit that connects the electrodes and a power source. When voltage is applied across the battery terminals, it causes a chemical reaction within the battery that separates lithium ions from their host material in the cathode.
These ions then migrate through an electrolyte medium towards the anode, where they react with electrons to form metallic lithium. At this point, energy stored in external power source is converted into chemical energy that can be used later when needed. Once fully charged, no more lithium ions can fit into the anode and all available sites are filled with metallic lithium.
Despite being efficient and long-lasting compared to other types of batteries, there are limitations on how fast or slow a Li-ion battery must be charged so as not to damage its components or cause overheating which may lead to explosion or fire accidents. Therefore it’s important for users to heed recommendations such as using only original charger and maintaining proper temperature limits during charging process so as not compromise performance or safety of their devices.
- Why can’t lithium battery be charged at low temperature
Lithium batteries are widely used in various electronic devices due to their high energy density and longer life span compared to other battery types. However, these batteries require specific temperature conditions for optimal performance. Charging lithium batteries at low temperatures can cause irreversible damage to them.
At low temperatures, the ions in the lithium battery move more slowly, making it difficult for them to reach their respective electrodes. The result of this slow movement is that the battery’s capacity decreases, and it takes longer for it to charge fully. If charging continues under these conditions, lithium plating may occur on the anode surface, which can further decrease its capacity and negatively affect its overall performance.
Therefore, it is recommended not to charge a lithium battery when the temperature falls below 0°C or above 45°C. It is also essential to keep your device away from extreme weather conditions as they may impact the performance of your device’s battery over time.
- Why avoid excessive discharge
Lithium batteries work by utilizing the movement of lithium ions from one electrode to another through a separator. During the discharge process, the lithium ions move from the negative electrode (anode) to the positive electrode (cathode), producing an electric current that powers our devices.
Excessive discharge occurs when a battery is drained beyond its recommended capacity. This can lead to irreversible damage and cause a decrease in performance or even complete failure of the battery. When a lithium battery is over-discharged, it can result in internal shorts, reduced capacity, and increased self-discharge rates.
Furthermore, excessive discharge can also pose safety risks such as overheating and explosion due to gas buildup inside the battery. Therefore, it’s crucial to avoid over-draining your lithium batteries and ensure they are charged before reaching critical levels of power. By doing so, you not only extend their lifespan but also keep yourself safe from any potential hazards associated with excessive discharge.
- What can we do if the battery pack is over-discharged
If a lithium battery pack is over-discharged, the first thing to do is to disconnect it from any charging source and remove it from the device. Leaving an over-discharged battery in a device can cause further damage or pose safety risks such as leakage, swelling, or even explosion. It’s important not to expose an over-discharged lithium battery to high temperatures or physical damage that can trigger thermal runaway and lead to a fire hazard.
Once you’ve isolated the over-discharged battery pack, you may try some recovery methods depending on the severity of the discharge and the type of battery chemistry used. For example, if your lithium-ion battery has a protection circuit built-in, you could apply a low-voltage charger that provides enough current to reactivate the protection circuit but not too much which could cause overheating. Another method is using an external power supply with adjustable voltage and current settings to gradually restore the voltage level until it reaches around 3V per cell.
However, keep in mind that these recovery methods may not always work or have potential risks associated with them. Therefore, prevention is key when it comes to avoiding over-discharging of your lithium batteries by monitoring their state-of-charge regularly and recharging them before they reach critical levels.
- What is battery balancing
Battery balancing is an important process that helps to maintain the health and longevity of lithium products. When multiple cells are connected in a battery pack, they can become unbalanced over time due to differences in their internal resistance and capacity. This can lead to some cells being overcharged or undercharged, which can cause them to degrade faster than others.
To prevent this from happening, battery balancing involves monitoring each cell’s voltage and adjusting the charging current as needed to ensure that all cells are charged equally. This is typically done using a specialized battery management system (BMS) that can detect imbalances and take corrective action.
By ensuring that all cells are charged and discharged evenly, battery balancing helps to prolong the lifespan of lithium batteries and prevent premature failure. It also helps to improve overall performance by maintaining consistent power output across the entire battery pack.
If your want to know more about useful pieces of information about lithium ion batteries, please feel free to visit Jutlithium’s Blog, you’ll like it!
Shenzhen Justlithiumbattery is a China battery manufacturer, which provides 12V batteries, 24V batteries, EV batteries, 48V rack battery, powerwall, portable batteries, UPS, and all kinds of lithium products. support OEM services, if you are looking for lithium manufacturer recently, you’ll not want to miss today.
Or if you want get more tips on how to choosing lithium battery and maintenance, you can also visit justlithium’s blog to discover more.